ISO 13485 Certification in Singapore – A Complete Guide for Medical Device Companies
ISO 13485 Certification in Singapore: Everything Medical Device Companies Need to Know
If you are involved in the medical device industry or planning to establish a company & enter into this market, you probably must have heard about ISO 13485 certification. This is one of the important standards in the medical device industry that is designed to help organisations across the globe to have a systematic approach in maintaining the quality in designing, manufacturing of product or supply of medical devices. But what exactly is ISO 13485 certification, and why is it so important for your organisation that operates in the medical device industry? How can you get ISO 13485 certification in Singapore & other major cities Singapore, Jurong, Woodlands, Bukit Timah, Changi, Clementi, Ang Mo Kio, Bedok and its process. Here we will answer all these questions and understand more about ISO 13485 standard & break down the topic into simple steps which is easy-to-understand.
What Is ISO 13485 Certification?
ISO 13485:2016 global quality standard for the medical device industry this standard provides a set of framework and guidelines related to quality management system (QMS) help companies deliver medical devices and related services consistently with high quality & safety to the customers while meeting legal & regulatory requirements as well.
Unlike ISO 9001, which is a generic quality management standard, ISO 13485 QMS focuses solely on medical devices, which helps organisations in maintaining the quality and safety of the product and also meet legal and regulatory compliance and manage risks related to the product. It requires organizations to adopt a process-oriented approach to quality management that covers every stage of the medical device lifecycle from initial product design stage till it reaches the customer.
Why Is ISO 13485 Important for Medical Devices?
The medical device industry is growing day by day and it is also one of the highly regulated sectors so maintaining quality and safety of the product becomes very important to stay competitive in the market. Implementing & getting ISO 13485 certification for medical devices for your company shows that your meets international quality standards and regulatory requirements. This certification is often a prerequisite in order to get approvals for exporting the products or entering new markets like EU, and it also helps gain customers and regulators trust in your product.
Countries across the globe including Singapore require the medical device manufacturing companies to hold ISO 13485 certification in order to sell or export the product overseas. So, is ISO 13485 mandatory for medical devices? In many cases, yes — especially if you want to compete globally & get more business.
This standard not only helps organisations meet all the legal and regulatory requirements but also helps companies to manage their risks related to product safety and its quality. This helps to prevent any kind of penalties that may occur due to non-compliance.
ISO 13485 not only helps the organisations to meet legal standards in regulatory compliance, but also helps companies in reducing risks related to product safety and quality and the penalties due to non-compliance. Implementing ISO 13485 helps to develop a culture of continuous improvement within the organisation & also help to mase sure that patient safety is always a top priority.
Who Needs ISO 13485 Compliance in Singapore?
If your business is involved in:
- Designing medical devices
- Manufacturing medical devices
- Supplying medical devices
- Providing related services like sterilization or packaging
Any company that is into manufacturing of medical devices can apply for ISO 13485 certification; this includes startups as well as established companies. Small companies often wonder & think, can small companies get ISO 13485 certified? The answer is yes! The standard can be implemented at any type of organisation with any scale; it provides a framework that is flexible & supports businesses of all sizes in maintaining consistent quality & safety in the product that they manufacture.
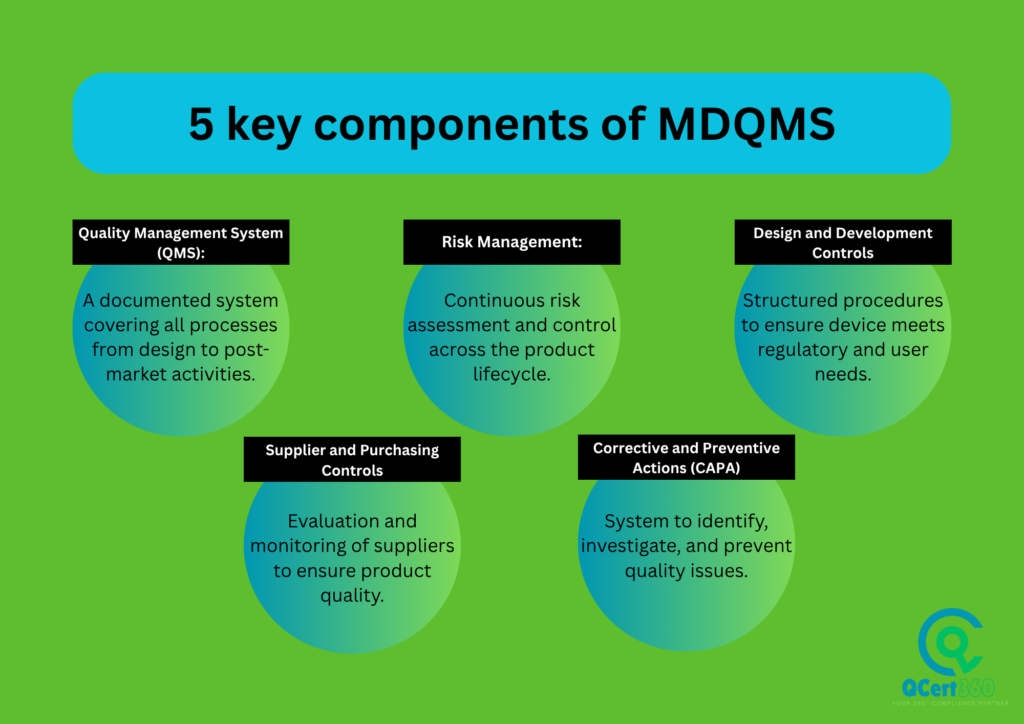
What Is ISO 13485 Certification Requirements?
To get your organisation ISO 13485 certified, it is important that your company must implement and follow the guidelines of quality management system for medical devices that meets all the requirements of the ISO 13485 standard. These requirements cover the following things:
- Documented processes and procedures (ISO 13485 documentation)
- Risk management throughout the product life cycle
- Design and development controls
- Supplier evaluation and control
- Traceability and product identification
- Corrective and preventive actions
- Internal audits and management reviews
- Regulatory compliance documentation
If you are a medical device startup, there are specific guidelines or ISO 13485 requirements for medical device startups to help them develop and establish the controls that are required to have a proper system in place from the beginning itself. Following the requirements early will help the startup to build a solid foundation and build trust with the customers and the regulatory bodies regarding the quality and safety of the product for the service that you are delivery
Steps to Implement ISO 13485 QMS in a Startup
Implementing ISO 13485 in a startup might be difficult initially, but breaking it down into clear steps helps & makes it easier:
- Understand the standard: it is important to understand & learn the core principles of ISO 13485 quality management system. Understand with the documentation and operational controls you will need.
- Gap analysis: conduct gap assessment to check your current processes against ISO 13485 requirements. Check & identify what are the things that you already have in place and what needs to be improved & generate a detailed gap assessment report with the findings.
- Implement controls: Put procedures in place for design control, supplier management, risk management, production processes, and quality checks.
- Train your team: Make sure everyone understands their roles & responsibilities in maintaining the quality management system within the organisation. Training your employees helps to maintain consistency in everyday activity and reduces any kids of errors.
- Create documentation: Develop documentation related to the ISO 13485 standard, which should include MDQMS policies, procedures related to your processes, and work instructions that are specific to your products and processes.
- Internal audits: Conducting regular checks helps to monitor & measure the effectiveness & performance of your system and fix if there are any issues. Internal audits help you prepare for your formal certification audit.
- Management review: Top management should review the QMS to make sure it is effective and there is continuous improvement.
- Prepare for certification audit: collect all necessary documents and evidence related to the standard to show your compliance. Practice answering auditors’ questions & to be confident.
For startups & the companies that are newly established, following these steps carefully can help them save time and money & avoid rework during the audit phase.
How to Get ISO 13485 Certification in Singapore?
In order to ISO 13485 certified usually organisations need to follows a clear process, led by a certification body. This is an accredited organization that checks and approves companies in Singapore for ISO 13485 compliance.
ISO 13485 Certification Process
- Select a certification body: Choose an ISO 13485 certification body or an ISO 13485 consulting company that is reputable & understands your industry & implements the standard in a proper manner.
- Pre-assessment audit (optional): Some companies choose to conduct a pre-assessment to identify if there are any gaps before the formal audit.
- Stage 1 audit: The auditors will review your documentation to check if it meets ISO 13485 standards & its guidelines.
- Stage 2 audit: A more detailed onsite audit is done to check & verify the implementation of your MDQMS.
- Certification issuance: If your organisation meets all requirements of the ISO 13485 standard, the certification body will issue ISO 13485 certificate to your company along with the detailed audit report.
- Surveillance audits: In order for an organisation to stay compliant with the ISO 13485 standard & maintain its certification, it is important for them to conduct or undergo surveillance audits regularly on time—once every year, failing to do so will lead to suspension of the certificate
What Documents Are Required for ISO 13485 Audit?
In order for an organisation to pass the ISO 13485 certification audit, it is important for them to develop detailed documentation related to the ISO 13485 standard, The list of documents includes:
- Quality manual
- Procedures and work instructions
- Records of training and competency
- Risk management files
- Design and development records
- Supplier evaluations
- Internal audit reports
- Management review minutes
- Corrective and preventive action (CAPA) records
developing and maintaining all these above documents helps an organisation to showcase their commitment to quality and safety because having proper documentation is very important as it makes things easier for an organisation to find out problems on time and make necessary improvements quickly to stay on track and be compliant over time.
How long does ISO 13485 certification take? ISO 13485 certification timeline
Usually, the ISO 13485 certification process takes around 3 to 6 months of time depending upon the size of the company and depending upon the complexity of the product that you’re manufacturing. Startups may take longer duration if they are starting from scratch.
How much does ISO 13485 Certification Cost? What are the things to consider?
The cost of ISO 13485 certification varies depending on several factors:
- Size and complexity of your organization
- Scope of certification (types of devices covered)
- Location (certification in Singapore or elsewhere)
- Need for consultancy services or pre-assessments
- Certification body fees
Smaller companies or startups can expect to spend less when compared to the larger companies with more complex operations or the product. Hiring MDQMS consultants can add up costs. But these consultants will help you to speed up the entire certification process and implement necessary best practices so that your organisation gets complaint. Always remember to invest in the right and knowledgeable consultant who can help you save money in the longer run and avoid any types of costly mistakes.
Implementing ISO 13485 can be difficult especially if you do not have any knowledge about the standard or how the quality management system is actually implemented into a company. This is where ISO 13485 certification consultants like Qcert360 come into play with their knowledge and expertise to help implement the standard to achieve compliance.
Hiring an ISO 13485 consulting company or specialists in the MDQMS consulting can provide you with:
- Expert guidance tailored to your business needs & requirements
- Conduct gap assessment
- Assistance in preparation of documentation related to the ISO 13485 standard
- Help in internal audits and training the staff
- Support during the certification audit
You do not have an idea on how to start the process and implement this standard into your system, taking assistance from the consultants can be invaluable. They can also help you understand & comply with local regulatory requirements, such as those specific to Singapore,
ISO 13485 Certification Service & Online Options
Today many certification bodies across the globe offer ISO 13485 certification online services to make things easier and process simpler for the companies that are looking to obtain the certification. The services provide audits remotely and conduct all the implementation activities via webinar sessions. This helps for companies that are facing travel restrictions or with global operations.
ISO 13485 Online certification services can reduce costs and let you work the way you want; this makes it easier for startups and small companies to get this certification while keeping their daily operations running smoothly without any breaks
What are the benefits of ISO 13485 Certification for Medical Device Companies
The advantages of getting ISO 13485 certification for your organisation are many & they go beyond just compliance. Some of the important advantages include:
- Improved quality and safety of the product
- Enhanced customer confidence and marketability
- Easier access to international markets and export approvals
- Streamlined internal processes and reduced risks related to safety and quality
- Compliance with legal & regulatory requirements (e.g., meeting CE marking requirements)
- Competitive edge in the market with your competitors with the non- ISO 13485 certified companies
Many buyers and vendors across the globe those who work in the regulated markets are interested to do business with the suppliers that have ISO 13485 certification. This certificate not only helps to meet the requirements of regulatory requirements but also builds a culture of quality and safety within the organisation which is very important for any business these days, because this can lead to better employee engagement and reduce the errors that may occur
ISO 13485 Accreditation and Registration
When a company achieves the certification, the ISO 13485 accreditation is usually issued by a certification body that is accredited & has affiliation with IAF, which can be either public or private depending on the certification body & its policies
This official recognition assures your customers and the regulatory bodies that you are quality management system meets international standards of quality and safety that are valid and accepted globally
How does the ISO 13485 Renewal and Maintenance process work?
ISO 13485 certification is not a one-time activity or an event. You must maintain compliance & regularly keep improving to keep your certification valid. ISO 13485 certification is usually valid for three years, but you need to undergo ISO 13485 renewal once in every 3 years & annually undergo surveillance audits every year to maintain compliance with the ISO 13485 standard & keep the status of the certification active.
How to Maintain ISO 13485 Compliance Year After Year? What are the things to follow
- Conduct internal audits within the organisation on a regular basis (at least once a year)
- Maintaining & keeping the documentation related to the standard up to date
- Address corrective actions promptly on a regular basis
- Perform management reviews regularly
- Monitor and improve your quality system continuously
Continuous improvement is one of the important aspects of the ISO 1345 quality management system. Companies that follow and do this well usually reduce the risk associated with quality of the product or service and improve customer satisfaction overtime.
How does the ISO 13485 Audit and Approval process work?
The MDQMS certification process usually involves a detailed ISO 13485 audit to be conducted for issuing the certificate. This is where the auditors will check every aspect of your quality management system. Only after successful completion of the audit is when your organisation will receive the ISO 13485 approval to go ahead & get a certificate of registration for your organisation.
It’s important to note that the auditors will just not focus on your documented procedures or your policies but they also check on how well these processes are being followed and are in practice. Therefore, it be important to provide employee trainings and awareness regularly
What is the important difference Between ISO 9001 and ISO 13485 standards?
ISO 9001:2015 is an international standard which is known for Quality Management System and this standard is applied to any type of business and suitable for any type of industry regardless of the nature of the activities they do.
ISO 13485:2016 is designed specifically for the companies that operate in the medical devices industry and this standard also includes additional requirements related to regulatory and risk management.
Many companies that work in the medical device industry choose to go ahead with ISO 13485 certification because it is specifically designed to directly align with their industry needs and regulatory requirements of the medical device industry.
How Does ISO 13485 Help Meet CE Marking Requirements?
For companies that are exporting their products to the European Union region, ISO 13485 standard plays a very important role & helps them in meeting the requirements of CE marking. This standard helps quality management systems align with the needs and requirements of the Devices Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) directives. Which are very important and the mandatory requirement if you are looking for CE marking approval and applying for a certification with a notified body.
Having ISO 13485 certification can help your organisation simplify your CE marking process because it provides evidence that are documented & provides compliance related to the quality and safety standards.
Who can issue ISO 13485 Certification in Singapore?
If you are looking to get ISO 13485 certification in Singapore, you should probably connect with local or international certification bodies that operate locally & have experience in the work they do & who can provide you the right solution. Medical device market is growing day by day in Singapore, and being ISO 13485 compliant is becoming very important for companies that work in this medical device manufacturing sector in order for them to meet the requirements of both local and export markets & get more business & recognition.
Is always important to work with a consultant or a certification body that has experience in the medical device industry and have enough knowledge & understands both local & international regulations and guidelines related to the medical device industry, because this can help you make the entire certification journey smooth.
Is ISO 13485 certification really important for your business in Singapore?
Getting ISO 13485 certification for your organisation is a very important step for any company that is working in the medical device industry or sector which is similar. It helps to make sure you meet global quality standards & meet all the legal & regulatory requirements to satisfy customer expectations. Whether you’re a startup or a well-established company, understanding how to get ISO 13485 certification & how exactly the ISO 13485 certification process works becomes very important, and also maintaining compliance year after year to stay compliant.
From choosing the right ISO 13485 certification body to getting expert guidance from ISO 13485 certification consultants also plays an important role in your achieving certification and enjoy the many benefits this standard offers.
If you’re ready to start your ISO 13485 certification journey & if you are looking for some who can provide you expert guidance in achieving this milestone, consider reaching out to professional ISO 13485 experts like Qcert360 who can provide ISO 13485 certification support at every step of the way.


