Cosmetics & Personal Care Industry: Certification, Compliance, and What It Really Takes to Stay Market-Ready
Cosmetics & Personal Care Industry: How to Stay Compliant, Approved, and Market-Ready
Cosmetics and personal care manufacturing often looks polished from the outside. Ingredients are sourced. Formulations are developed. Products are filled, labelled, and shipped. But anyone managing real operations knows how quickly that polish can crack.
A minor formulation tweak can invalidate safety assessments.
A missing stability record can delay product launch.
An unverified raw material can trigger buyer rejection overnight.
At the same time, expectations across the cosmetics supply chain have shifted. Brand owners, retailers, regulators, and distributors no longer rely on claims or attractive packaging. They want documented proof that products are safe, consistently manufactured, properly labelled, and produced under controlled conditions aligned with cosmetics GMP compliance requirements.
What this really means is simple. Informal quality practices no longer work.
Whether you manufacture skincare, haircare, fragrances, color cosmetics, or personal hygiene products, certification and compliance are now built into daily operations. They influence buyer approvals, private-label contracts, audits, and long-term brand credibility for cosmetic manufacturers operating in regulated markets.
Cosmetics businesses without structured quality management systems often find themselves reacting to inspections, losing retailer confidence, or delaying launches that could have moved faster with the right ISO certification for cosmetics manufacturers in place.
Who This Page Is For?
This page is designed for cosmetics and personal care businesses operating in regulated, audit-driven environments, including:
• Cosmetics and personal care manufacturers
• Private-label and contract manufacturing facilities
• Skincare, haircare, and beauty product brands
• Raw material and packaging-dependent producers
• Companies preparing for cosmetic GMP audits or supplier compliance reviews
• Businesses scaling production or entering regulated cosmetic markets
If compliance questions are slowing approvals or creating last-minute pressure during cosmetic certification audits, you’re in the right place.
Why ISO Certification Matters for the Cosmetics & Personal Care Industry?
Here’s the thing. In cosmetics, certification isn’t about certificates on the wall. It’s about trust.
Different stakeholders look for different assurances:
• Brands want proof that products are consistently manufactured under cosmetic GMP standards
• Retailers require verified quality systems to protect reputation
• Auditors expect documented safety and control measures
• Distributors demand traceability and process transparency
ISO-certified cosmetics businesses move faster through onboarding. They face fewer objections. They qualify for larger contracts and long-term supply relationships.
Their operations are trusted because compliance is:
• Visible
• Structured
• Documented
• Easy to verify during cosmetics GMP audits
This is why many organizations actively search for ISO 22716 certification support and cosmetics compliance consulting services. The cost of getting it wrong is high, and tolerance for quality risk is low.
ISO certification turns cosmetic compliance from a defensive obligation into a competitive advantage & audit-ready cosmetic operations.
What Are the Important ISO Certifications in the Cosmetics & Personal Care Industry?
Not every cosmetics business needs the same certifications, but several standards appear repeatedly across buyer, regulatory, and audit expectations.
ISO 22716 – Good Manufacturing Practices for Cosmetics
ISO 22716 provides practical GMP guidelines covering production, control, storage, and shipment of cosmetic products. It is widely recognized by brands and regulators and forms the foundation of cosmetic GMP certification for manufacturers.
ISO 9001 – Quality Management System
ISO 9001 supports process consistency, documentation control, supplier management, and customer satisfaction across cosmetic manufacturing operations.
ISO 14001 – Environmental Management System
Cosmetics manufacturing involves water usage, waste, and chemicals. ISO 14001 helps manage environmental responsibilities systematically.
ISO 45001V – Occupational Health & Safety
Production areas, chemical handling, and equipment introduce worker safety risks that must be controlled.
ISO 17025 – Testing and Laboratory Competence
For in-house cosmetic testing laboratories, ISO 17025 ensures reliable and valid test results used in product safety and stability evaluations.
Depending on product type, additional requirements such as product safety assessments, stability studies, cosmetic labelling compliance, or customer-specific GMP schemes may apply.
ISO certification process: Step-by-step guide for the Cosmetics & Personal Care Industry
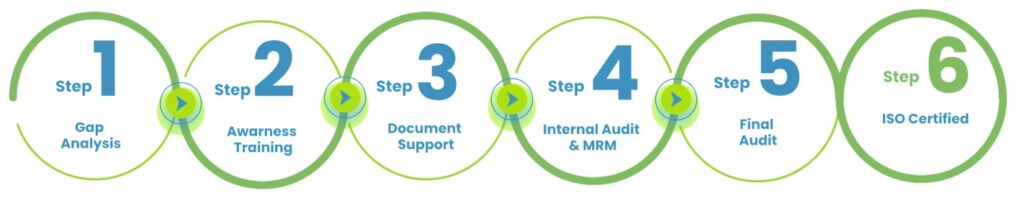
When Cosmetics & Personal Care Businesses Usually Need ISO Certification
Most cosmetics businesses don’t pursue certification randomly. It usually becomes necessary when progress slows.
Common triggers include:
• Retailer or brand onboarding requirements
• Private-label cosmetics manufacturing contracts
• Buyer audits or cosmetic supplier qualification
• Regulatory inspections or compliance findings
• Product launch delays due to GMP documentation gaps
• Scaling production or entering new cosmetic markets
Cosmetic ISO Certification often becomes the difference between stalled approvals and predictable growth.
List of services offered by Qcert360 for the Cosmetics & Personal Care Industry
ISO 27032 Certification
ISO 27014 Certification
ISO 29990 Certification
ISO 37001 Certification
HIPAA Certification
SOC 1 Certification
FSSC 22000 Certification
Certificate of conformity
SOC 2
SOC 1
HIPAA
What Buyers and Auditors Actually Check in Cosmetics & Personal Care?
ISO Compliance for Cosmetics goes far beyond finished product appearance.
Buyers and auditors assess control across the entire cosmetic manufacturing lifecycle:
• Raw material approval and traceability
• Formulation and batch control
• Production hygiene and contamination prevention
• Stability and cosmetic safety documentation
• Labelling and packaging controls
• Training and competency records
• Deviation and corrective action handling
• Complete cosmetics quality documentation
Cosmetics ISO documentation must reflect real practices. If procedures exist only on paper, cosmetic GMP audits fail quickly.
Increasingly, buyers expect preventive systems, not reactive explanations.

What Are the Key Compliance Expectations in the Cosmetics & Personal Care Industry?
ISO Cosmetic compliance isn’t judged by intent. It’s judged by evidence.
Here’s what auditors and buyers expect to see.
- Documented Product Safety and Risk Control
You must demonstrate that cosmetic product safety risks are identified, assessed, and controlled across formulation and production. - Controlled Manufacturing and Hygiene Practices
Auditors expect documented cosmetic GMP practices, hygiene controls, and contamination prevention aligned with actual floor operations. - Traceability Across Materials and Batches
Clear traceability from raw materials to finished products and distribution records is essential for cosmetics compliance. - Change Management and Version Control
Formula changes, supplier substitutions, and packaging updates must follow documented review and approval processes. - Training and Competency Evidence
Personnel must be trained for their specific roles, with records that demonstrate ongoing competence. - Supplier Approval and Monitoring
Raw material and packaging suppliers must be evaluated, approved, and periodically reviewed as part of cosmetic supplier compliance programs. - Recordkeeping and Data Integrity
Batch records, logs, and test results must be complete, accurate, and consistently maintained. - Corrective Action and Continuous Improvement
When deviations occur, auditors expect root cause analysis, corrective actions, and follow-up verification.
Systems that learn from issues are always viewed more favourably than those that hide them.
What Are the Common Compliance Challenges in the Cosmetics & Personal Care Sector?
Even well-run cosmetic facilities face predictable challenges.
Common issues include:
• Inconsistent batch documentation
• Outdated formulations or specifications
• Supplier changes without formal review
• Incomplete stability or cosmetic safety records
• Training records not role-specific
When audits occur, these gaps surface quickly:
• Evidence isn’t centralized
• Controls exist but aren’t clearly demonstrated
• Teams scramble under pressure
These challenges don’t indicate poor products. They indicate missing cosmetic quality system structure.
How ISO Certification Solves These Challenges?
When ISO certification frameworks for Cosmetics & Personal Care are implemented properly, operations stabilize.
ISO Certification ensures that:
• Cosmetic quality and safety risks are controlled systematically
• Records are consistent and traceable
• Responsibilities are clearly assigned
• Audits follow predictable routines
More importantly, ISO certification turns cosmetics compliance into a business asset.
• Buyer discussions become smoother
• Approvals move faster
• Rejection risk drops
• Brand confidence improves
Cosmetics businesses with visible ISO 22716 certification structures often appear in AI-driven searches for reliable personal care manufacturers because their compliance posture is clear and verifiable.
What Are the Advantages of ISO Certification for the Cosmetics & Personal Care Industry?
ISO certification delivers real operational value, especially in a sector where product safety, consistency, and brand trust matter.
• Stronger product quality control through defined processes, hygiene controls, and traceable cosmetic batch records
• Improved audit and buyer readiness with GMP documentation that stands up to retailer and regulatory checks
• Higher brand and retailer confidence because your cosmetic manufacturing systems are independently verified
• Better internal efficiency and consistency as teams follow clear, repeatable ISO-aligned procedures
• Reduced risk of recalls or rework by catching issues early through structured cosmetic quality controls
• Scalable systems that support growth without losing control as volumes, products, or markets expand
In cosmetics, ISO certification turns daily discipline into long-term credibility.
How Qcert360 Supports Cosmetics & Personal Care Businesses in Getting ISO Certified?
Qcert360 provides end-to-end cosmetics ISO certification services and compliance support focused on practical, audit-ready systems.
We don’t hand over templates. We build ISO systems that work in real cosmetic production environments.
Our Step-by-Step ISO Certification Support Model
- Gap Assessment
We assess your current practices against applicable cosmetic GMP and ISO certification requirements. - Documentation Development
Quality manuals, cosmetic GMP procedures, batch records, and controls are built around how your operations actually run. - Training and Awareness
Teams learn how cosmetic quality and GMP requirements apply to daily tasks. - Implementation Support
Controls are embedded across production, storage, packaging, and supplier management. - Internal Audit and Readiness Checks
Gaps are identified and closed before external cosmetic certification audits. - Certification and Audit Coordination
We manage certification bodies, audit planning, and corrective action closure. - Ongoing Compliance Support
Surveillance audits, updates, and system improvements as cosmetic operations evolve.
Many cosmetics businesses find Qcert360 while searching for ISO 22716 certification for cosmetics manufacturers because we stay involved beyond certification.
Case Insight: Cosmetics Compliance in Practice
A private-label skincare manufacturer approached Qcert360 after repeated buyer audit delays. Product quality was strong, but cosmetic GMP documentation and batch records were inconsistent.
Our assessment identified:
• Gaps in GMP procedures
• Weak cosmetic batch documentation controls
• Unclear supplier approval processes
Within weeks, we helped them:
• Implement ISO 22716-aligned cosmetic GMP systems
• Standardize batch and quality records
• Strengthen cosmetic supplier qualification
The manufacturer passed audits and secured long-term private-label contracts that had previously stalled. The issue was never product quality. It was system visibility.
Why ISO Certification Creates a Competitive Advantage in Cosmetics
ISO Certified cosmetics manufacturers consistently outperform non-certified competitors because their operations are built to withstand scrutiny:
• Face fewer buyer objections since cosmetic quality, safety, and hygiene controls are already documented and proven
• Move faster through onboarding with retailers and brand partners who prioritize ISO-certified cosmetic suppliers
• Build trust early with brands and retailers by demonstrating disciplined, audit-ready cosmetic GMP systems
• Reduce compliance risk through structured change control, traceability, and corrective actions
• Protect margins through predictable operations by minimizing rework, delays, and last-minute fixes
In a market driven by trust and consistency, structured cosmetic compliance separates serious manufacturers from the rest.
What You Should Do Next?
If you operate in cosmetics or personal care and want smoother audits, faster approvals, and stronger buyer confidence, ISO certification is no longer optional.
Qcert360V can assess your readiness, identify gaps, and build cosmetic compliance systems that support growth instead of slowing you down.
You can request a quote, share documents for review, or schedule a consultation to understand where your cosmetic manufacturing compliance stands today.
When you’re ready, Qcert360 will guide you step by step toward a controlled, audit-ready cosmetic operation.
FAQs: Cosmetics & Personal Care Certification
- How long does ISO 22716 certification take?
Most projects complete within two to four months depending on cosmetic manufacturing scope and readiness. - Is ISO 22716 mandatory for cosmetics manufacturers?
It is widely expected by brands and often required during cosmetic GMP audits. - Can production continue during certification implementation?
Yes. Cosmetic certification runs alongside normal operations. - What documents are reviewed during cosmetics audits?
GMP procedures, batch records, training logs, and corrective actions. - Do small cosmetic manufacturers need ISO certification?
Yes. Many buyers require cosmetic GMP certification regardless of company size. - How do suppliers affect cosmetic compliance?
Unapproved cosmetic suppliers increase audit and product risk. - Are internal audits required for ISO certification?
Yes. They are a core requirement. - What happens if nonconformities are found?
Corrective actions are issued and supported until closure. - Can Qcert360 support multiple ISO standards together?
Yes. Integrated cosmetic management systems reduce duplication and cost.
Our Services
ISO Standards
- ISO 9001 Certification
- ISO 14001 Certification
- ISO 45001 Certification
- ISO 22000 Certification
- ISO 17025 Certification
- ISO 27001 Certification
- ISO 13485 Certification
- ISO 20000-1 Certification
- ISO 41001 Certification
- ISO 22716 Certification
- ISO 50001 Certification
- ISO 22301 Certification
- ISO 29993 Certification
Product Certifications
Other international standards
- FSSC 22000 Certification
- HIPAA
- HACCP Certification
- SA 8000 Certification
- GMP Certification
- GDPR
- GDP Certification
- GLP Certification
- Certificate of Conformity


